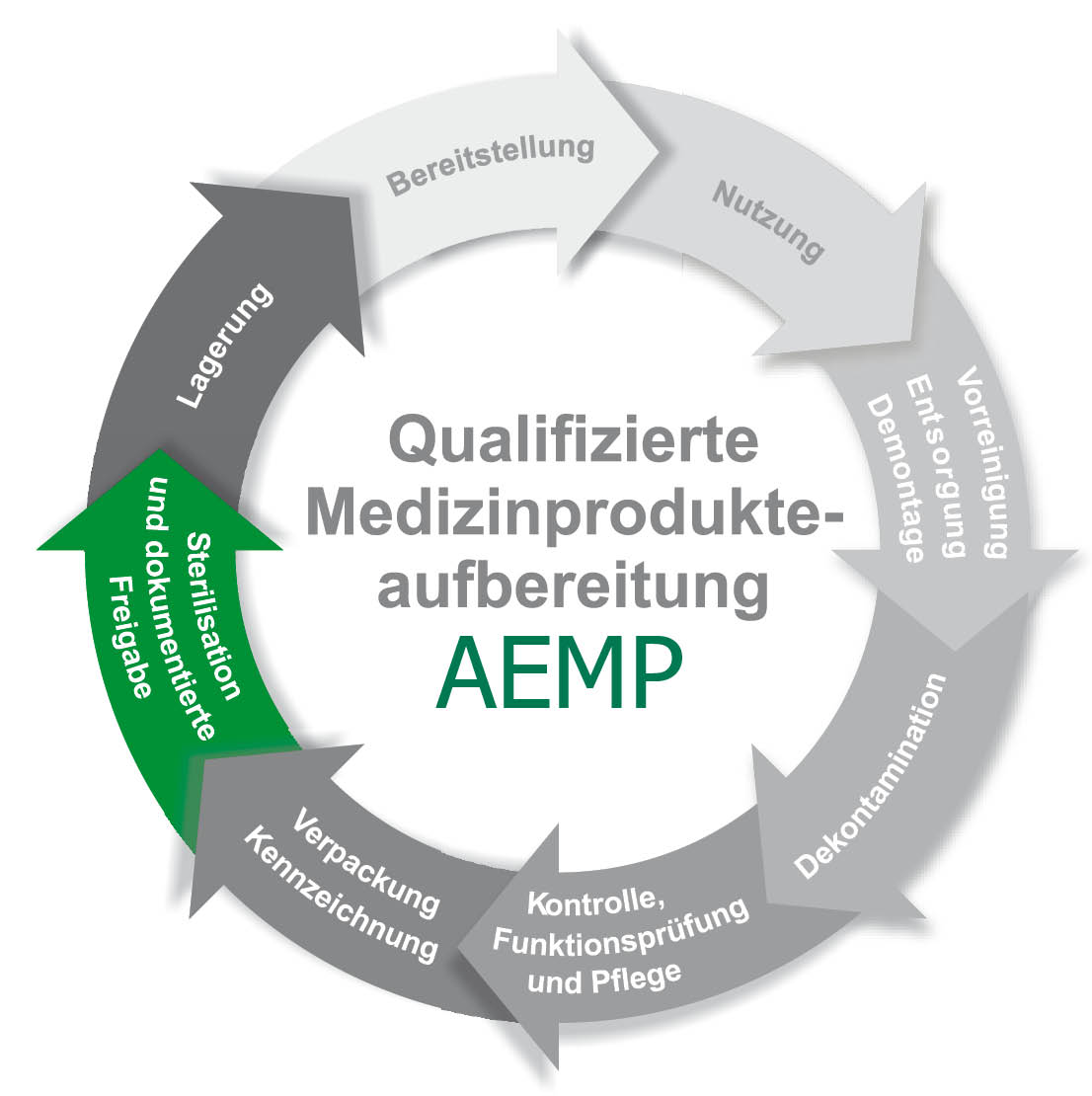
The core task is usually the supply of reprocessed work equipment. In addition, the functional departments and wards of a hospital can also be supplied. As a rule, the entire production process of reprocessing medical devices is carried out in the CSSD. Today, medical devices are divided into non-critical, semi-critical, critical and with or without increased reprocessing requirements. It is important to achieve the highest possible documentation density in order to have the respective good under control at all times, which serves not only to safeguard against complaints but also to ensure patient safety.
Instrumentcycle Packaging with colour indicators to check sterilisation success Supply of the sets
Surgical instruments are prepared in sets, which can be packaged in various forms (soft or hard packaging). The imprecise, rather historical term sieve is also commonly used. Most common are sterile goods containers in all variations and sizes below a sterile goods unit, which are increasingly being replaced by baskets wrapped in fleece.
In a sterile supply store, the sets are kept and stored until they are used. They are labelled with an expiry date. It is important to regularly check, replenish and update the stock so as not to store dead stock.
Cleaning
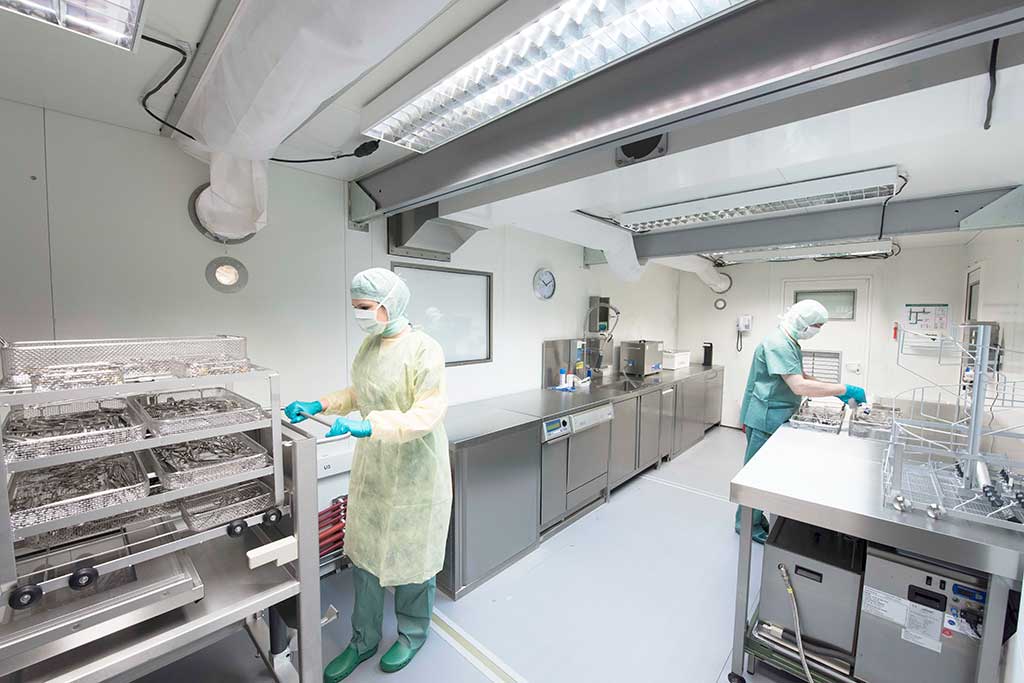
After an operation, the used surgical instruments are taken to the unclean area of the CSSD, where they are disassembled as far as possible according to the manufacturer’s instructions, if not already done in the operating room. Hard-to-reach areas are pre-cleaned using plastic brushes, steam cleaners or ultrasound, if necessary. Further cleaning is done in the washer-disinfector. Uniform cleaning can only be achieved by automated cleaning processes. From a global perspective, however, instruments are mostly cleaned manually.
Disinfection
The material is then placed in a washer-disinfector. It works in a similar way as a dishwasher, but unlike a dishwasher, it has special washing programmes with different cleaning agents. The goods are cleaned in it, then thermally disinfected and finally dried. The aim is to minimise pre-cleaning as much as possible, on the one hand to achieve consistent results time and time again, and on the other hand to save time. Time, rinsing mechanics, cleaning chemistry and temperature are the effective parameters in the sense of Sinner’s circle that determine the success of the cleaning.
Control, Care and Packing
On the clean side of the CSSD, the washer-disinfectors are emptied. They should be set up in such a way that they act as pass-through machines separating the unclean from the clean area. The goods are now checked for cleaning success and functionality, sorted and, where necessary, treated with special, steam-permeable instrument care spray to prevent wear. Packing lists, ideally recorded in an EDP system, specify how the finished set is to be packed. They consist of an inventory list and a topographical allocation so that the instruments are always positioned in the same way in the container.
Sterilisation
The packed set is now sterilised according to the manufacturer’s instructions. As a rule, in about 90 % of cases, the steam sterilisation process is used; this is referred to as autoclaving. In the CSSD, gas sterilisation with ethylene oxide, formaldehyde steam and so-called plasma sterilisation are also used. The advantage here is that the goods can be sterilised at lower temperatures, which is indicated for thermolabile materials. The aim is to kill all microorganisms. After sterilisation, the sterile goods must be released, i.e. by an employee who has at least completed the course „Specialist Knowledge I“ and who must then check the goods or the packaging for any damage and document a release. The course for „Specialist Knowledge I“ can be acquired from many training providers. It is considered the minimum entry qualification for this occupational area. Many hospitals, reprocessing units such as CSSDs, medical and dental practices and external service providers employ staff who have completed it.