CSSD as a Modular System
Comfortable, air-conditioned container CSSD
Dust-protected, thermally insulated and prepared for cold as well as warm climates
HP Medizintechnik GmbH
CSSD as a Modular System
HP Medizintechnik GmbH offers standard-compliant reprocessing units for medical devices (AEMP) specially designed for flexible and mobile use.
For the military, non-governmental organisations (NGOs) or as an interim solution for hospitals.
Our mobile CSSDs are all exclusively equipped with an internal treatment of deionised water.
They require only one drinking water inlet and one wasterwater outlet.
- The systems are resource-minimised.
- They have very low water and power consumption.
- They are designed for operating in different climatic zones.
40 years of know-how in sterilisation technology
Maximum security despite exceptional mobility
The mobile CSSD ensure a very fast reaction time.
Required on site:
- 1 Week for set-up, integration, installation and approval
- 1 Week for commissioning and disinfection


Mobile CSSDs delivered to the German Armed Forces were deployed in Afghanistan (Kabul and Kunduz), in Kosovo (Prizren) and in Mali, partially under extreme conditions.
A reproducible, validatable cleaning, disinfection and sterilisation process is implemented in the AEMP. A cleaning and disinfection area (unclean area), a packing and sterilisation area (clean area) and a personnel lock are used accordingly.
The modules offer spacious work areas and high-quality equipment with cleaning equipment and devices for disinfection, self-sufficient steam sterilisers as well as systems for the production of partially demineralised and fully demineralised water.


The convenient mobile CSSD, housed in an air-conditioned container, is dust-protected, thermally insulated and equipped for cold as well as warm climates.
A comprehensive validation concept for temporary mobile use ensures consistently high quality at all times and regardless of location.
Mobile reprocessing of medical devices of the highest quality
The systems are very flexible, can be set up quickly, are resource-optimised and can be operated self-sufficiently. The adapted special packaging concepts have already proven themselves in use in cold as well as warm climatic zones.
The special packaging allows the CSSDs to be transported by land, sea and air. A container that can be docked to the packing and sterilisation area is equipped with stainless steel cabinets for the long-term storage of sterile goods.
In addition, the systems are also prepared for extreme environmental conditions through design measures, e.g. during air transport (frost resistance) or during storage and operation.


The modules offer spacious working areas and high-quality equipment, with washer-disinfectors, self-sufficient steam sterilisers and systems to produce partially demineralised and fully demineralised water.
Short, intersection-free paths support and guarantee an optimal workflow and ensure efficient work steps as well as low stress for the operating staff.
Access to the “cleaning and disinfection area” and “packing and sterilisation area” occurs via a staff passage.
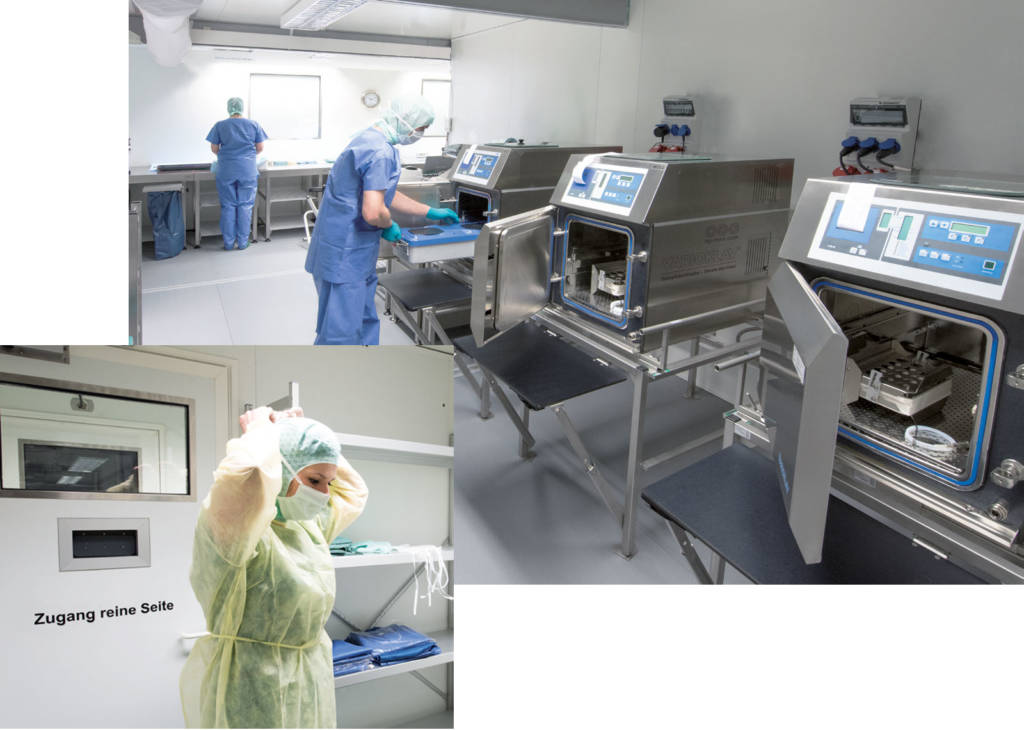
Our AEMP system in action
Hygiene requirements
It complies with the “Hygiene Requirements for the Reprocessing of Medical Devices of the Commission for Hospital Hygiene and Infection Prevention (KRINKO) at the Robert Koch Institute (RKI) and the Federal Institute for Drugs and Medical Devices (BfArM)”.
Conformity
It thus enables reprocessing of “thermostable medical devices” in conformity with laws and standards (Critical A and B according to “DGSV on the classification of medical devices 2013”).
Quality
Outstanding product quality is part of our self-image. Therefore, our quality system ensures continuous improvement of our processes in all areas of the company.
Feel free to contact us
You need technical help or have a query?
Send us a message! We will contact you as soon as possible.
Telephone
+49 (89) 45 35 19 4 -50
info@hp-med.com
Opening hours
9am – 4pm

